Which listing has the orbitals in order of increasing energy in a multielectron atom?
A) 3s 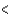 3p
3p 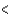 4s
4s 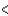 3d
3d 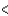 4p
4p
B) 3s 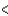 3p
3p 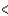 3d
3d 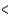 4s
4s 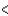 4p
4p
C) 3s 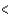 4s
4s 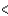 3p
3p 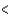 4p
4p 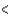 3d
3d
D) 3s 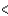 3px
3px 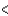 3py
3py 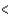 3pz
3pz
E) 2s 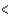 3s 2p
3s 2p 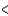 3d
3d
Correct Answer:
Verified
Q110: What is the ground-state electron configuration of
Q111: Which of the following elements has the
Q112: Two elements that have np3 in their
Q113: What is the ground-state electron configuration of
Q114: Which of the following orbitals is the
Q116: Which orbital does not fill before a
Q117: Which of the following elements has the
Q118: Which transition metal ion has no d
Q119: Which atom or ion has the largest
Q120: What is the ground state electron configuration
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents