Give the names of the three quantum numbers (n, 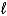 , and m
, and m 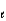
) that identify the mathematical functions (orbitals) that are solutions to Schrödinger's wave equation, and describe how the energy, size, shape, and orientation of the orbital varies with the relative values of these quantum numbers.
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q155: Astronomers have detected hydrogen atoms in interstellar
Q156: De Broglie proposed that
Q157: `If cesium, which has a work function
Q158: Recently, buckyballs (C60) became the largest objects
Q159: Write the electron configuration for each of
Q161: Define effective nuclear charge. Use this parameter
Q162: Arrange the following elements in order of
Q163: Arrange the following ions in order of
Q164: Arrange the following elements in order of
Q165: Arrange the following elements in order of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents