A hot air balloon, under the conditions shown below, experiences an increase in pressure. What will the new volume of the balloon be? 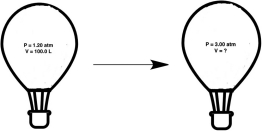
A) 3.6 10-2 L
B) 12 L
C) 28 L
D) 40 L
E) 250 L
Correct Answer:
Verified
Q34: If the pressure of a system is
Q35: A 25.0 L balloon is filled with
Q36: A sample of Ar gas at
Q37: Which of the following graphs shows the
Q38: An unknown gas held in a 25.0
Q40: What is the volume of a gas
Q41: In an experiment, 7.5 mol of
Q42: Considering that PV = nRT, which
Q43: Which of the following reactions will result
Q44: The pressure gauge on a 100-L cylinder
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents