Which of the gases shown here will exert the lowest pressure, assuming that each container has the same volume and temperature?
A) 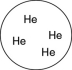
B) 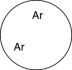
C) 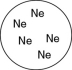
D) 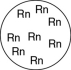
Correct Answer:
Verified
Q16: The pressure of a gas is inversely
Q17: Two identical cement blocks are placed on
Q18: A 1.25 L bottle of soda exerts
Q19: What pressure (in Pa) will be
Q20: A balloon is filled with 3.00 L
Q22: The temperature of a gas is directly
Q23: Which of the following graphs shows the
Q24: A sample of gas at 4.0
Q25: Four containers, each with the same volume
Q26: A 1.50 mol sample of He occupies
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents