The pressure gauge on a cylinder used to fill balloons with He shows a pressure of 1,275 psi at a temperature of 25 C. Assuming the cylinder has a volume of 10 L, how many grams of He does the cylinder contain?
A) 520 g
B) 142 g
C) 6.70 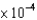 g
g
D) 37.0 g
E) 4.00 g
Correct Answer:
Verified
Q45: How many moles of propane are
Q46: What volume is occupied by 1.00
Q47: Which one of these samples contains
Q48: Calcium carbonate (100.1 g/mol) decomposes according to
Q49: Which of the following reactions will
Q51: What is the temperature if 3.0
Q52: Which of the gas samples shown below
Q53: Considering that PV = nRT, which
Q54: What is the pressure of a
Q55: Automobile air bags inflate during a crash
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents