Calcium carbonate (100.1 g/mol) decomposes according to the following reaction equation: CaCO3(s)  CaO(s)
CaO(s) 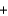 CO2(g)
CO2(g)
What pressure of CO2 gas will form in a 25.0 mL jar containing 5.00 g CaCO3 at 300 K if 0.00500% of the solid decomposes?
A) 0.246 atm
B) 2.46 atm
C) 1.00 atm
D) 2.46 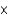 103 atm
103 atm
E) 2.46 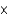 10-3 atm
10-3 atm
Correct Answer:
Verified
Q43: Which of the following reactions will result
Q44: The pressure gauge on a 100-L cylinder
Q45: How many moles of propane are
Q46: What volume is occupied by 1.00
Q47: Which one of these samples contains
Q49: Which of the following reactions will
Q50: The pressure gauge on a cylinder
Q51: What is the temperature if 3.0
Q52: Which of the gas samples shown below
Q53: Considering that PV = nRT, which
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents