Before the development of reliable batteries, miners' lamps burned acetylene produced by the reaction of calcium carbide with water. A typical lamp used 0.75 L of acetylene per hour at 1.00 atm pressure and 20°C. How many grams of calcium carbide and how many grams of water had to be in the lamp for a 4-hour shift?
CaC2(s) 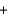 H2O(l)
H2O(l)  C2H2(g)
C2H2(g) 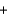 CaO(s)
CaO(s)
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q139: As an ideal gas, 1 mol
Q140: A rectangular gold block has a mass
Q141: The density of a gaseous compound of
Q142: A gas has a density of 2.40
Q143: What is the temperature if 3.0 g
Q145: Hyperbaric oxygen chambers providing pure oxygen (O2)
Q146: Which one of these samples contains
Q147: A scuba diver releases a balloon containing
Q148: Three bulbs are connected as shown in
Q149: Oxygen for astronauts in space can
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents