Assuming that the distances between the two ions are the same in all cases, which of the following ion pairs has the smallest electrostatic potential energy (i.e., smallest in magnitude) ?
A) 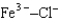
B) 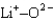
C) 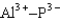
D) 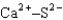
E) 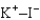
Correct Answer:
Verified
Q4: The kinetic energy associated with the random
Q5: Work is done when a force moves
Q6: Work requires _
A)a use of potential energy.
B)a
Q7: The capacity to do work is a
Q8: What is the kinetic energy of a
Q10: Heat is best defined as _
A)a substance
Q11: Assuming that the distance between ions remains
Q12: Assuming that the charge of the ions
Q13: A diver jumps off a diving board.
Q14: According to Coulomb's law, which ionic compound
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents