Which statement A-D about the first law of thermodynamics  is not correct?
is not correct?
A) (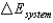 is the change in the internal energy of a system.)
is the change in the internal energy of a system.)
B) q is the energy added to or removed from the system.
C) q usually is called heat.
D) w is the energy added to or removed from the system by a deformation; that is, a force moves something.
E) The statements A-D are all correct.
Correct Answer:
Verified
Q28: Which statement A-D about a system and
Q29: During a(n) _ process, energy is transferred
Q30: Which of the following is an endothermic
Q31: If a chemical reaction causes the temperature
Q32: During a(n) _ process, energy is transferred
Q34: Which one of the following statements is
Q35: A student dissolving some ammonium nitrate (NH4NO3)
Q36: The following diagrams illustrate the flow of
Q37: Which arrow in the following diagrams represents
Q38: The following diagrams illustrate the flow of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents