You heat a cup of coffee in a microwave oven. It absorbs 40 kJ of energy from the microwave, and the volume expands. Which one of the following statements correctly describes the relationship between the change in enthalpy H and the change in internal energy E of the coffee? (> means greater than, < means less than)
A) ( H  40 kJ, E
40 kJ, E 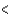 40 kJ)
40 kJ)
B) ( H  40 kJ, E
40 kJ, E 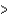 40 kJ)
40 kJ)
C) ( H 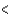 40 kJ, E
40 kJ, E  40 kJ)
40 kJ)
D) ( H 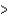 40 kJ, E
40 kJ, E  40 kJ)
40 kJ)
E) ( H  40 kJ, E
40 kJ, E  40 kJ)
40 kJ)
Correct Answer:
Verified
Q40: Which of the following bar charts shows
Q41: A cooling curve for some substance is
Q42: The change in enthalpy and the change
Q43: Which statement A-D about the first
Q44: A cooling curve for some substance is
Q46: Which statement below regarding the various heat
Q47: How much work does a gas do
Q48: The best definition of the enthalpy change
Q49: In a steam engine, steam in a
Q50: What is the heat capacity (Cp)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents