The heating curve for a substance is shown below. The substance initially is a solid. It then becomes a liquid and a gas. Which of the line segments (I-V) represents the solid-to-liquid phase transition? 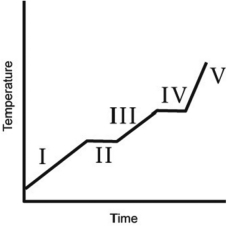
A) I
B) II
C) III
D) IV
E) V
Correct Answer:
Verified
Q47: How much work does a gas do
Q48: The best definition of the enthalpy change
Q49: In a steam engine, steam in a
Q50: What is the heat capacity (Cp)
Q51: At a certain elevation, the boiling
Q53: A pot of water is heated
Q54: Which statement A-D about energy units is
Q55: What is the change in internal
Q56: What will be the final temperature
Q57: During an exothermic process, _ for
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents