Use the following information to determine the enthalpy for the reaction shown below.
S(s) + O2(g) SO2(g) H = ?
S(s) 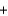
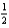 O2(g)
O2(g)  SO3(g)
SO3(g) 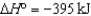 2SO2(g)
2SO2(g) 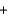 O2(g)
O2(g)  2SO3(g)
2SO3(g) 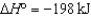
A) (-593 kJ)
B) (+593 kJ)
C) (-296 kJ)
D) (+296 kJ)
E) (-989 kJ)
Correct Answer:
Verified
Q77: You have a summer job in a
Q78: In an experiment, 74.3 g of
Q79: Using the following data for water, determine
Q80: In an experiment, 7.5 g of
Q81: Isooctane is a good model compound
Q83: The integrated circuits in your cell
Q84: Isooctane is a good model compound for
Q85: Which statement regarding combustion of a sample
Q86: Napthalene is often used to determine
Q87: Which substance has an enthalpy of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents