In terms of the enthalpy of formation, which of the following compounds is most stable relative to its elements under standard conditions?
A) PbBr2(s) , 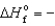 277.4 kJ/mol
277.4 kJ/mol
B) O3(g) ,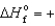 142.3 kJ/mol
142.3 kJ/mol
C) SO2(g) , 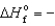 269.9 kJ/mol
269.9 kJ/mol
D) H2Se(g) , 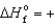 29.7 kJ/mol
29.7 kJ/mol
E) ICl(g) , 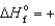 17.8 kJ/mol
17.8 kJ/mol
Correct Answer:
Verified
Q83: The integrated circuits in your cell
Q84: Isooctane is a good model compound for
Q85: Which statement regarding combustion of a sample
Q86: Napthalene is often used to determine
Q87: Which substance has an enthalpy of
Q89: When a 13.0 g sample of
Q90: Use the following information to determine
Q91: Indicate which of the following is not
Q92: Which statement regarding combustion of a sample
Q93: Predict the temperature change produced by
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents