Determine the enthalpy for the following reaction, given
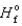 (NH3(g) ) = - 46.1 kJ/mol,
(NH3(g) ) = - 46.1 kJ/mol,
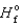 (NO(g) ) =
(NO(g) ) = 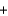 90.3 kJ/mol, and
90.3 kJ/mol, and 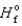 (H2O(g) ) = -241.8 kJ/mol. 4NH3(g)
(H2O(g) ) = -241.8 kJ/mol. 4NH3(g) 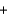 5O2(g)
5O2(g)  4NO(g)
4NO(g) 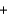 6H2O(g) Hrxn = ? kJ
6H2O(g) Hrxn = ? kJ
A) (-1274 kJ/mol)
B) (-1,996 kJ/mol)
C) (+1,274 kJ/mol)
D) (-905.2 kJ/mol)
E) (-105.4 kJ/mol)
Correct Answer:
Verified
Q107: Fuel value is _
A)the cost of energy
Q108: Explain what is meant by the term
Q109: In a Reuters news report dated
Q110: The enthalpy of combustion of table sugar
Q111: Suppose you eat a one-third pound hamburger
Q113: Determine the standard enthalpy of formation
Q114: Given the standard enthalpies of formation
Q115: In a Reuters news report dated February
Q116: Ethanol (CH3CH2OH) has been suggested as
Q117: Which of the following hydrocarbons has the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents