Sports trainers use cold packs containing ammonium nitrate for injured athletes. Calculate the change in temperature when 47 g of ammonium nitrate (NH4NO3, 80.1 g/mol) dissolves in 100 g water. Assume the specific heat of the solution is 4.5 J/(g . C).
NH4NO3(s)  NH4+(aq)
NH4+(aq) 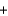 NO3-(aq) H = 21.1 kJ/mol
NO3-(aq) H = 21.1 kJ/mol
Correct Answer:
Verified
Q119: Ethanol (CH3CH2OH) has been suggested as
Q120: Which of the following fuels has the
Q121: Benzoic acid is used to determine
Q122: Given the standard enthalpies of formation
Q123: The basal metabolic rate (BMR) is the
Q125: The complete combustion of 2.500 g
Q126: Determine the enthalpy for the following
Q127: To cool your 250 mL of
Q128: Use the following information to determine
Q129: State Hess's law as it applies to
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents