What mass of potassium iodide (molar mass = 166.0 g/mol) is needed to produce 325.0 mL of a solution that has a concentration of 0.0150 M ?
A) 2.94 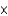
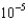 g
g
B) 0.809 g
C) 809 g
D) 7.66 g
E) 1.31 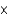
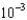 g
g
Correct Answer:
Verified
Q23: The World Health Organization recommends that the
Q24: Commercial hydrochloric acid is 12.1 M. What
Q25: Which of the following compounds is a
Q26: Which picture best represents an atomic-level view
Q27: What volume of 3.0 M NaOH contains
Q29: How many moles of ammonium hydroxide are
Q30: Which contains more solute particles: a 0.10
Q31: How many grams of solid magnesium chloride,
Q32: How many moles of magnesium nitrate are
Q33: Which picture best represents an atomic-level view
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents