The World Health Organization recommends that the maximum allowable concentration of chromium(VI) in drinking water be limited to 0.05 mg/L. If the molar absorptivity of chromium(VI) at 345 nm is 1.5 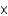 103/(M cm) , what is the absorbance of a solution that contains 0.050 mg/L in a 10-cm-long cell?
103/(M cm) , what is the absorbance of a solution that contains 0.050 mg/L in a 10-cm-long cell?
A) 0.0014
B) 0.75
C) 0.014
D) 0.075
E) 14
Correct Answer:
Verified
Q18: If 120 g of NaOH were used
Q19: Which of the following cartoons depicts a
Q20: A medical saline solution is prepared by
Q21: If 100 mL of 3.0 M solution
Q22: What volume of 12 M HCl solution
Q24: Commercial hydrochloric acid is 12.1 M. What
Q25: Which of the following compounds is a
Q26: Which picture best represents an atomic-level view
Q27: What volume of 3.0 M NaOH contains
Q28: What mass of potassium iodide (molar mass
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents