Identify the acid in the following acid-base reaction.
PbCO3(s) 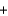 H2SO4(aq)
H2SO4(aq)  PbSO4(s)
PbSO4(s) 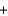 CO2(g)
CO2(g) 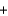 H2O(l)
H2O(l)
A) PbCO3(s)
B) CO2(g)
C) PbSO4(s)
D) H2O(l)
E) H2SO4(aq)
Correct Answer:
Verified
Q53: Which of the following compounds is a
Q54: In the following reaction, H2O _ CH3COOH(aq)
Q55: Which one of the following is the
Q56: In a demonstration of strong electrolytes, weak
Q57: Which one of the following reaction equations
Q59: Which one of the following statements regarding
Q60: Chalk contains calcium carbonate. What would be
Q61: Which of the following phosphate compounds is
Q62: If 1.0 L of 1.0 M HCl
Q63: A 500 mg dietary supplement of L-lysine
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents