Sodium thiosulfate (Na2S2O3, molar mass = 158.2 g/mol) is used in the development of photographic film. Your summer job is at a photography lab and you need to check the purity of an outdated supply. You react 40.21 mL of 0.246 M iodine solution with a 3.232 g sample. What is the percent purity of the sodium thiosulfate that you report to your boss?
I2(aq) +2S2O32-(aq)  2I-(aq)
2I-(aq) 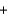 S4O62-(aq)
S4O62-(aq)
A) 100%
B) 48.4%
C) 96.8%
D) 98.6%
E) 84.4%
Correct Answer:
Verified
Q68: Which of the following compounds are soluble
Q69: Which one of the following is not
Q70: Hard water contains Mg2+ and Ca2+ ions
Q71: Which of the following ionic compounds is
Q72: How many liters of 0.200 M NaOH
Q74: Which of the following ionic compounds is
Q75: Most alkaline earth salts are soluble. Identify
Q76: Which one of the following water solubility
Q77: Water-soluble toxic chromium compounds are waste products
Q78: Ammonia (NH3) is a weak base that
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents