In going from the top to the bottom of the activity series below, the ease of oxidation of the metal ________, which means that ________. 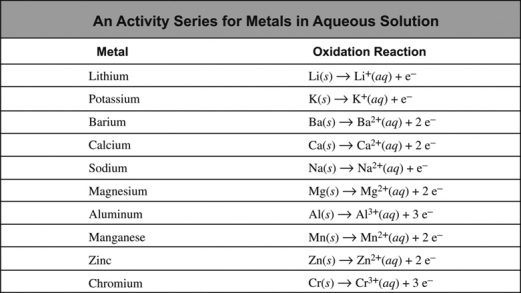
A) increases; magnesium can reduce zinc ions
B) increases; magnesium ions can oxidize zinc
C) decreases; zinc can reduce magnesium ions
D) increases; zinc ions can oxidize magnesium
E) decreases; magnesium can reduce zinc ions
Correct Answer:
Verified
Q113: In which compound does chlorine have an
Q114: Groundwater often has low levels of dissolved
Q115: The half-reaction for the reduction of molecular
Q116: In a spontaneous oxidation reduction reaction between
Q117: Based on the activity series shown below,
Q119: What is the oxidation number of P
Q120: The concentration of iron(II) ions in water
Q121: Write the balanced molecular, complete ionic, and
Q122: Draw pictures that represent: (a) a
Q123: A food chemist determined the concentration of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents