Identify the species that is oxidized and reduced in the following oxidation-reduction reaction.
Fe2O3(s) 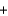 3CO(g)
3CO(g)  2Fe(s)
2Fe(s) 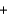 +3CO2(g)
+3CO2(g)
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q138: Write the net ionic equation for the
Q139: Why is an aqueous solution of table
Q140: The drinking water standard of the
Q141: Identify the oxidation number of the nonoxygen
Q142: Controlling the ammonia and ammonium ion concentrations
Q143: Some microorganisms living under anaerobic conditions extract
Q144: Controlling the concentration of ammonia is
Q145: Identify the oxidizing and reducing agents in
Q147: Identify which of the following compounds are
Q148: Based on the activity series shown below,
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents