One mole is defined as ________
A) the number of particles equal to the number of atoms in exactly 12 g of carbon-12.
B) the number of particles equal to the number of atoms in exactly 16 g of oxygen-16.
C) exactly 6.02 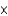 1022 particles.
1022 particles.
D) the number of particles equal to the number of atoms in exactly 1 g of hydrogen-1.
E) the number of particles equal to the number of atoms in exactly 1 kg of carbon in a vault at the National Bureau of Standards.
Correct Answer:
Verified
Q1: A particular manufacturer sells phosphoric acid capsules
Q2: In one analysis, 3.01 Q3: Which one of the following samples contains Q4: How many hydrogen atoms are there in Q6: A gallon of water has a mass Q7: How many moles of ammonia are there Q8: Which statement about the following chemical reaction Q9: In one analysis, the density of ozone Q10: For a standard airbag, 131 g of Q11: A sample of water (H2O) contains 1.81![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents