Hydrogen Peroxide Decomposes to Produce Water and Oxygen 2H2O + O2
A)2 Molecules 2 Molecules +1 Molecules
Hydrogen peroxide decomposes to produce water and oxygen. Which relationship regarding the quantities of reactants and products associated with this reaction is not correct?
2H2O2 2H2O + O2
A) 2 molecules  2 molecules +1 molecules
2 molecules +1 molecules
B) 34.0 g  18.0 g
18.0 g 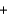 16.0 g
16.0 g
C) 68.0 g  36.0 g
36.0 g 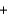 32.0 g
32.0 g
D) 2x mol  2x mol
2x mol 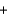 x mol
x mol
E) y(34.0 g)  y(18.0 g)
y(18.0 g) 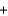 y(32 g)
y(32 g)
Correct Answer:
Verified
Q18: In one analysis, 1.34 Q19: How many gold atoms are there in Q20: A tiny speck (8.3 Q21: How many mL of hexadecane (C16H34) are Q22: Which statement about the following chemical Q24: Fuming sulfuric acid is obtained by the Q25: Air bags in cars inflate when an Q26: Sulfur dioxide from coal-fired power plants combines Q27: Fe2O3(s) and powdered aluminum can react with Q28: Nitrogen monoxide undergoes combustion to produce nitrogen![]()
![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents