Phosphorus trichloride reacts with water to form phosphorous acid, H3PO3, and hydrochloric acid in the following unbalanced reaction. For each mole of phosphorous acid produced by this reaction, how many moles of HCl are produced?__ PCl3 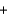 __H2O
__H2O  __H3PO3
__H3PO3 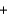 + __ HCl
+ __ HCl
A) 1
B) 2
C) 3
D) 4
E) 5
Correct Answer:
Verified
Q39: How many oxygen atoms are there in
Q40: Identify the list below that has these
Q41: The combustion of ethanol (CH3CH2OH, 46.1 g/mol)
Q42: In a demonstration of the complete combustion
Q43: The average adult exhales about 1.0 kg
Q45: Respiration is to photosynthesis as _
A)a lake
Q46: Which statement A-D regarding photosynthesis is not
Q47: Balance the following combustion reaction and report
Q48: What are the stoichiometric coefficients for oxygen
Q49: Diesel fuel for automobiles and trucks is
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents