Identify the set of stoichiometric coefficients that balances the reaction equation for the combustion of octane. C8H18 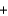 O2
O2  CO2
CO2 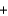 H2O
H2O
A) 1, 25, 8, 9
B) 1, 17, 8, 9
C) 2, 34, 16, 18
D) 2, 25, 16, 18
E) None of the above coefficients balance the reaction equation.
Correct Answer:
Verified
Q51: If the combustion of fossil fuels
Q52: Ozone (O3) reacts with iodide (I-) and
Q53: Which statement A-D regarding the carbon cycle
Q54: One form of elemental sulfur is a
Q55: The combustion of fossil fuels is the
Q57: Ammonia undergoes combustion to produce nitrogen monoxide
Q58: Glucose (C6H12O6) is oxidized by molecular oxygen
Q59: A fuel cell works on the same
Q60: Burning coal that contains sulfur releases sulfur
Q61: An iron ore, magnetite, contains only iron
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents