Baking soda (NaHCO3, 84.0 g/mol) requires acids from other ingredients to generate the carbon dioxide needed to make bread rise. The following equation describes this reaction, where HB is some unspecified acid. If 20.4 g of baking soda are used in a recipe and enough acid is present for a complete reaction, how many moles of carbon dioxide are generated? HB 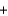 NaHCO3
NaHCO3  H2O
H2O 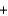 CO2
CO2 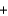 NaB
NaB
A) 0.464 mol
B) 0.334 mol
C) 0.243 mol
D) 0.204 mol
E) 0.232 mol
Correct Answer:
Verified
Q68: Arizona was the site of a 400,000-acre
Q69: A range of organic molecules can undergo
Q70: Water can be separated into its elements
Q71: Even though lead is toxic, lead compounds
Q72: What mass of phosphoric acid (H3PO4, 98.0
Q74: The average car emits 8.0 kg
Q75: The most abundant metal in Earth's crust
Q76: Fool's Gold is the mineral pyrite (FeS2).
Q77: Elemental analysis of the soot produced by
Q78: Elemental analysis of the organic compound meta-xylene
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents