Copper sulfate is a blue solid that is used to control algae growth. Solutions of copper sulfate that come in contact with the surface of galvanized (zinc-plated) steel pails undergo the following reaction that forms copper metal on the zinc surface. How many grams of zinc would react with 454 g (1 lb) of copper sulfate (160 g/mol) ?
CuSO4(aq) 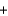 Zn(s)
Zn(s)  Cu(s)
Cu(s) 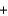 ZnSO4(aq)
ZnSO4(aq)
A) 467 g
B) 186 g
C) 93 g
D) 234 g
E) 454 g
Correct Answer:
Verified
Q61: An iron ore, magnetite, contains only iron
Q62: Metallic sodium was originally produced by
Q63: Copper was the first metal to be
Q64: Baking ammonia or ammonium bicarbonate (NH4HCO3, 79.1
Q65: One form of asbestos called chrysotile is
Q67: A compound known to contain platinum, nitrogen,
Q68: Arizona was the site of a 400,000-acre
Q69: A range of organic molecules can undergo
Q70: Water can be separated into its elements
Q71: Even though lead is toxic, lead compounds
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents