A reaction vessel contains equal masses of solid magnesium metal and oxygen gas. The mixture is ignited and burns with a burst of light and heat, producing solid MgO. The mass of the MgO is less than the initial mass of the magnesium and oxygen. What is your explanation for this apparent loss of mass?
A) Conservation of mass is violated in this reaction.
B) Some of the mass was converted into energy (heat and light) as 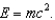
C) Not all of the oxygen reacted.
D) Not all of the magnesium reacted.
E) Measurement must be in error because mass is conserved in chemical reactions.
Correct Answer:
Verified
Q100: Nicotine is an alkaloid and a component
Q101: A copper ore consisting of 12.5% copper(II)
Q102: Beryllium metal reacts with chlorine gas to
Q103: At the Tesla automotive factory, a Model
Q104: U.S. Lime & Minerals is a company
Q106: At Sal's Sandwich Shop, a regular club
Q107: Write a definition of the term molar
Q108: In which of the following reaction mixtures,
Q109: What mass of phosphoric acid (H3PO4, 98.00
Q110: How many total atoms are there in
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents