In a self-contained breathing apparatus, potassium superoxide, KO2, reacts with carbon dioxide to form potassium carbonate and oxygen. Identify the limiting reactant and determine the amount of oxygen gas, in moles, that can be produced from 250 g of KO2 and 450 g of CO2.
4KO2(s) 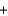 2CO2(g)
2CO2(g)  2K2CO3(s)
2K2CO3(s) 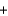 3O2(g)
3O2(g)
A) potassium superoxide limiting, 4.75 mol
B) potassium superoxide limiting, 1.53 mol
C) potassium superoxide limiting, 2.64 mol
D) carbon dioxide limiting, 1.53 mol
E) carbon dioxide limiting, 2.64 mol
Correct Answer:
Verified
Q106: At Sal's Sandwich Shop, a regular club
Q107: Write a definition of the term molar
Q108: In which of the following reaction mixtures,
Q109: What mass of phosphoric acid (H3PO4, 98.00
Q110: How many total atoms are there in
Q112: For movie night, Annabelle popped two bags
Q113: Calcium hydride (CaH2) is so reactive with
Q114: A bottle containing 1,665 g of sulfuric
Q115: Dialuminum hexachloride, Al2Cl6 (266.66 g/mol), is an
Q116: Household bleach contains 5.0% sodium hypochlorite (NaOCl)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents