One reaction that occurs during the deployment of an air bag is shown below, where sodium metal reacts with potassium nitrate. If 5.10 g of sodium and 305 g of potassium nitrate react in an airbag, how many grams of KNO3 remain because of the limited amount of sodium present?
10Na(s) 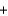 2KNO3(s)
2KNO3(s)  K2O(s)
K2O(s) 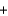 5Na2O(s)
5Na2O(s) 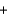 N2(g)
N2(g)
A) 2.24 g
B) 112 g
C) 193 g
D) 301 g
E) 303 g
Correct Answer:
Verified
Q112: For movie night, Annabelle popped two bags
Q113: Calcium hydride (CaH2) is so reactive with
Q114: A bottle containing 1,665 g of sulfuric
Q115: Dialuminum hexachloride, Al2Cl6 (266.66 g/mol), is an
Q116: Household bleach contains 5.0% sodium hypochlorite (NaOCl)
Q118: Ammonia, NH3, is an important industrial chemical.
Q119: A mass of 11.60 g of phosphoric
Q120: What is the molar mass of glucose,
Q121: Calculate the mass percent of each element
Q122: Write the balanced reaction equation for the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents