Like many metals, manganese reacts with halogens to give metal halides. How many grams of MnF3 can be produced using excess fluorine and 5.49 g of Mn? (molar masses: Mn = 54.9 g/mol,
F  19.0 g/mol)
19.0 g/mol)
2Mn(s) 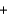 3F2(g)
3F2(g)  2MnF3(s)
2MnF3(s)
Correct Answer:
Verified
Q121: Calculate the mass percent of each element
Q122: Write the balanced reaction equation for the
Q123: For which of the following compounds are
Q124: Which of the following oxides of nitrogen
Q125: Oranges derive their distinct smell from a
Q127: Metallic sodium was originally produced by
Q128: Without doing any calculations, identify which of
Q129: Diborane undergoes combustion according to the following
Q130: A burner in a gas grill mixes
Q131: What is the difference in the chemistry
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents