The Dieterici equation of state, 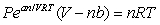 , gives the relationship between pressure P, volume V, and temperature T for a liquid or gas. At the critical point,
, gives the relationship between pressure P, volume V, and temperature T for a liquid or gas. At the critical point, 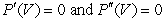 with T constant. Using the result of the first derivative and substituting it into the second derivative, find the critical volume Vc in terms of the constants n, a, b, and R.
with T constant. Using the result of the first derivative and substituting it into the second derivative, find the critical volume Vc in terms of the constants n, a, b, and R.
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q58: Find the second-degree polynomial (of the form
Q59: Use the graph of Q60: Let Q61: Find an equation of the line tangent Q62: Compute the derivative of Q64: The function Q65: Find the derivative of Q66: Find the derivative of Q67: Find the derivative where f is an Q68: Differentiate the function. Unlock this Answer For Free Now! View this answer and more for free by performing one of the following actions Scan the QR code to install the App and get 2 free unlocks Unlock quizzes for free by uploading documents![]()
![]()
![]()
![]()
![]()
![]()
![]()

