Consider the following reaction at equilibrium: CO₂ + H₂O 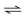 H₂CO₃. What would be the effect of adding additional H₂O?
H₂CO₃. What would be the effect of adding additional H₂O?
A) It would drive the equilibrium dynamics to the right.
B) It would drive the equilibrium dynamics to the left.
C) Nothing would happen because the reactants and products are in equilibrium.
D) Reactions in both the directions will slow down.
Correct Answer:
Verified
Q28: What is the pH of a solution
Q31: Q32: Q32: A 0.01 M solution of a substance Q33: How much of 0.5 M glucose (molecular Q34: How many grams of the compound in Q35: Carbon dioxide in the atmosphere dissolves with Q37: A solution contains 0.0000001 (10-7) moles of Q38: Identical heat lamps are arranged to shine Q41: A slice of pizza has 500 kcal.![]()
![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents