Carbon dioxide (CO₂) is readily soluble in water, according to the equation CO₂ + H₂O ↔ H₂CO₃. Carbonic acid (H₂CO₃) is a weak acid. If CO₂ is bubbled into a beaker containing pure, freshly distilled water, which of the following graphs correctly describes the results?
A) 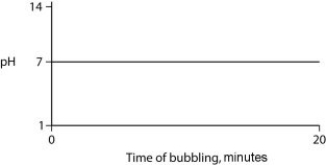
B) 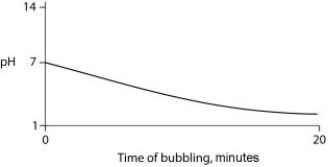
C) 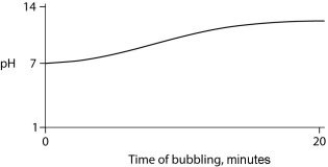
D) 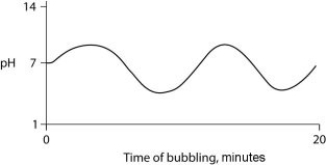
Correct Answer:
Verified
Q41: We can be sure that a mole
Q42: Measurements show that the pH of a
Q46: Assume that acid rain has lowered the
Q46: If the cytoplasm of a cell is
Q47: Measurements show that the pH of a
Q47: How would acidification of seawater affect marine
Q50: As the [H3O+] of the solution decreases,
Q52: Which of the following is a hydrophobic
Q52: A beaker contains 100 millilitres (mL) of
Q54: Increased atmospheric CO₂ concentrations might have what
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents