Which one of the atoms shown would be most likely to form an anion with a charge of -1?
A) 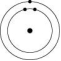
B) 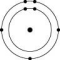
C) 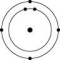
D) 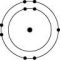
Correct Answer:
Verified
Q17: Which of the following are compounds?
A) H₂O,
Q19: How many electrons will a single atom
Q21: Which one of the atoms shown would
Q21: Which of the following is broken when
Q22: A covalent bond is likely to be
Q23: What is the maximum number of hydrogen
Q26: Nitrogen (N) is more electronegative than hydrogen
Q26: Bonds between two atoms that are equally
Q28: The atomic number of chlorine is 17.
Q36: Van der Waals interactions may result when
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents