Instructions: Refer to the data below to answer the following question(s).
of Some Phenols 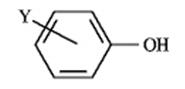
Refer to instructions.
a) The weakest acid in the table is
b) Which of the acids in the table has the weakest conjugate base?
Correct Answer:
Verified
Q9: Instructions: Provide correct IUPAC names for each
Q10: Instructions: Draw structures corresponding to each of
Q11: Instructions: Provide correct IUPAC names for each
Q12: Instructions: Draw structures corresponding to each of
Q13: Instructions: Consider the Grignard reaction below to
Q15: Instructions: Draw structures corresponding to each of
Q16: Which of the following would not react
Q17: Instructions: Consider the Grignard reaction below to
Q18: Instructions: To answer the following question(s), consider
Q19: Instructions: Consider the Grignard reaction below to
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents