To answer the questions below, consider the following information:
In an abandoned laboratory has been found a flammable liquid, A, in a bottle bearing only the label "Compound A: C7H12. Government agents have offered you a considerable sum to determine the structure of this compound. After verifying the molecular formula by elemental analysis, you find that Compound A reacts with 1 mol equiv of hydrogen and, after treatment with acidic KMnO4, gives the dicarboxylic acid C (see below). Another bottle from the same laboratory is labeled "Compound B (isomer of A)." Compound B also reacts with 1 mol equiv of hydrogen, but yields cyclohexanone after treatment with acidic KMnO4. 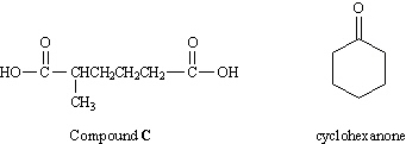 What was the other product formed in the KMnO4 oxidation of B?
What was the other product formed in the KMnO4 oxidation of B?
Correct Answer:
Verified
Q33: What type of reactive intermediate is formed
Q34: Draw all the isomeric products formed
Q35: Complete the following reaction
Q36: Draw two resonance structures for the species
Q37: Draw two resonance structures for the species
Q38: Instructions: Predict the products of each reaction
Q39: What type of reaction mechanism accounts for
Q41: Povidone is produced commercially as a series
Q43: Lyapolate Sodium, whose structure is shown below,
Q44: MATCH each definition to the term it
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents