Below is the mass spectrum of an unknown hydrocarbon. In addition, this hydrocarbon shows characteristic absorption at 2100 cm-1 in its IR spectrum. Give the structure of this unknown. 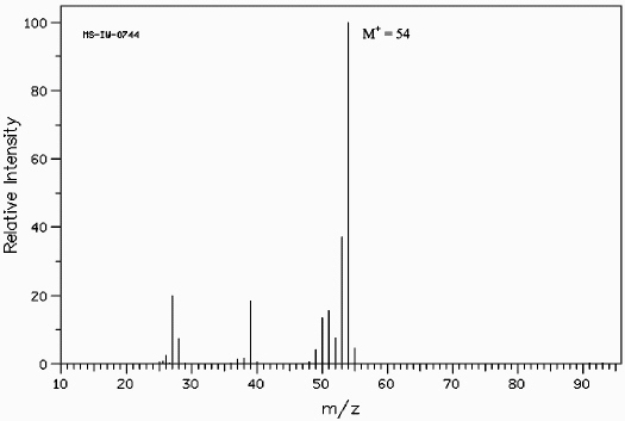 (Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/)
(Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/)
The formula weight of 54 corresponds to a molecular formula of C4H6, which has two degrees of unsaturation. Possible structures for this formula are:
Correct Answer:
Verified
Q1: Instructions: Match each of the following groups
Q6: What are the units for electromagnetic radiation
Q7: Cyclohexene and hex-2-yne both have the molecular
Q8: At what approximate positions might the compound
Q9: The amount of energy in electromagnetic radiation
Q10: Instructions: Refer to the mass spectrum of
Q11: Assume you are carrying out the conversion
Q12: Which of the following bonds undergoes stretching
Q20: Which of the following bonds undergoes stretching
Q22: Examining the infrared spectrum of a compound
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents