Enzymes in plants convert nitrate into ammonia. The unbalanced half-reaction equation is given below. What is the change in oxidation number of nitrogen in this reaction? NO3-(aq) + H2O( 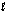 ) + e- NO2-(aq) + OH-(aq)
) + e- NO2-(aq) + OH-(aq)
A) 0
B) +5
C) +3
D) -2
E) -3
Correct Answer:
Verified
Q2: Nickel-cadmium batteries thrown into landfills can introduce
Q25: Which of the following is not a
Q31: Plants convert nitrogen into ammonia. This
Q35: Urea is a waste product produced when
Q38: Plants react urea with water to produce
Q50: Adding fluoride ions to toothpaste and drinking
Q55: Adding fluoride ions to toothpaste and drinking
Q56: Hydroxyapatite, which is a component of
Q63: The isotope 201Th is used in the
Q78: Shape memory alloys are used in _
A)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents