Hearing Aid Batteries Utilize a Zinc/air Electrochemical Cell Zn(s) + 2OH-(aq)
-1)249 V
O2(g) + 2H2O( )
Hearing aid batteries utilize a zinc/air electrochemical cell. The reduction half-reactions and standard reduction potentials for this cell are given below. What species are oxidized and reduced in the cell reaction? Zn(OH) 2(s) + 2e- Zn(s) + 2OH-(aq)
-1) 249 V
O2(g) + 2H2O( 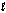 ) + 4e- 4OH-(aq)
) + 4e- 4OH-(aq)
+0) 401 V
A) Oxygen is reduced and zinc is oxidized.
B) Zinc is reduced and oxygen is oxidized.
C) Zinc hydroxide is reduced and oxygen is oxidized.
D) Zinc hydroxide is reduced and hydroxide is oxidized.
E) Water is reduced and zinc is oxidized.
Correct Answer:
Verified
Q24: Calcium carbonate is used to form exoskeletons.
Q38: Enzymes in plants convert nitrate into
Q40: Plants convert nitrogen into ammonia. This
Q42: Iron accumulation in the human body has
Q44: The average concentration of calcium in the
Q45: Hearing aid batteries utilize a zinc/air
Q46: Copper is a trace metal in the
Q54: Chromium is an ultratrace metal in
Q65: Technetium can be produced in a nuclear
Q69: Pacemakers implanted in the chests of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents