Hearing Aid Batteries Utilize a Zinc/air Electrochemical Cell Zn(s) + 2OH-(aq)
-1)249 V
O2(g) + 2H2O( )
Hearing aid batteries utilize a zinc/air electrochemical cell. The reduction half-reactions and standard reduction potentials for this cell are given below. Write the balanced reaction equation for the cell and report the sum of the stoichiometric coefficients. Zn(OH) 2(s) + 2e- Zn(s) + 2OH-(aq)
-1) 249 V
O2(g) + 2H2O( 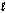 ) + 4e- 4OH-(aq)
) + 4e- 4OH-(aq)
+0) 401 V
A) 4
B) 5
C) 6
D) 7
E) 8
Correct Answer:
Verified
Q4: One reason some metals have toxic effects
Q30: How much energy must be expended by
Q34: The concentration of sodium in the human
Q51: What is the dominant role played by
Q53: Different concentrations of ions on either
Q55: Hearing aid batteries utilize a zinc/air
Q58: Fluoridation to prevent tooth decay replaces _
Q58: The concentration of Li in the
Q59: Hearing aid batteries utilize a zinc/air
Q67: Radioactive tritium, 3H, is used as
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents