Urease is an enzyme that catalyzes the decomposition of urea to ammonia and carbon dioxide. The rate constant for the uncatalyzed reaction is 4.0 *10-11/s at 22°C. Urease increases the rate constant to a value of 8.0 *10-5/s. Use the Arrhenius equation, which is given below, to determine the difference in the activation energies Euncatalyzed - Ecatalyzed. (R = 8.315 J/mol K) 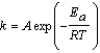
Correct Answer:
Verified
Q81: Draw two possible Lewis structures of the
Q82: Why are the radioactive isotopes of cesium
Q85: Hearing aid batteries utilize a zinc/air
Q86: Plants convert nitrogen into ammonia. This
Q89: Fluoride is added to toothpastes and drinking
Q92: The photosynthesis of carbohydrates from carbon dioxide
Q93: What kinds of metals typically are found
Q94: An enzyme catalyzes the decomposition of hydrogen
Q94: A patient is injected with a
Q95: Hydroxyapatite, which is a component of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents