An electrochemical cell is constructed with a zinc metal anode in contact with a 0.052 M solution of zinc(II) nitrate and a silver cathode in contact with a 0.0042 M solution of silver(I) nitrate. What is the emf of this cell at 5°C? Metal/Metal ion
E 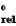 silver/silver(I) +0.799 V
silver/silver(I) +0.799 V
Zinc/zinc(II) -0.762 V
A) 1.656 V
B) 1.465 V
C) 1.561 V
D) 1.370 V
E) 1.609 V
Correct Answer:
Verified
Q34: The electrodes on batteries are labeled +
Q35: The unit of electrical power, watt (W),
Q38: The unit of current, Ampere (A), is
Q42: A voltaic cell is constructed based on
Q43: A voltaic cell is constructed based on
Q55: If the potential of a voltaic cell
Q65: Consider the voltaic cell based on
Q89: An electrochemical cell has both a silver
Q124: For a chemical reaction to be considered
Q127: How do fuel cells differ from traditional
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents