Does pH have an effect on the cell potential (Ecell) for the following oxidation-reduction reaction? 5Fe2+(aq) + MnO4-(aq) + 8H+(aq) 5Fe3+(aq) + Mn2+(aq) + 4H2O( 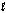 )
)
A) Yes, because Ecell values for all redox reactions depend on the pH.
B) Yes, because Ecell values for redox reactions involving the hydronium ion depend on the pH.
C) No, because Ecell values for redox reactions depend only on the major species in the reaction, in this case, Fe2+, MnO4-, Fe3+, and Mn2+.
D) No, because Ecell values for redox reactions depend on concentrations and temperatures but not on pH.
E) No, because Ecell values for redox reactions do not depend on the pH.
Correct Answer:
Verified
Q10: Which one of the following items does
Q24: The work involved in moving exactly 1
Q27: A concentration cell is constructed by using
Q56: Which statement does not correctly describe
Q62: The magnitude of the charge on
Q62: Which statement does not correctly describe a
Q65: The spontaneous redox reaction in a
Q79: An electrochemical cell with a standard hydrogen
Q94: In a classroom demonstration of a redox
Q107: The charge supplied by a battery can
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents