The oxidation of hydrogen by oxygen is one of the most-used reactions in fuel-cell technology. The overall reaction, which is given below, has a G° value of -474 kJ/mol. What is the standard cell potential for this fuel cell? 2H2(g) + O2(g) 2H2O( 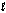 )
)
A) 2.46 V
B) 4.91 V
C) 1.23 V
D) 2.46 v
E) 1.50 V
Correct Answer:
Verified
Q36: The electrodes on batteries are labeled +
Q42: A voltaic cell is constructed based on
Q43: A voltaic cell is constructed based on
Q48: The oxidation of hydrogen by oxygen
Q50: Luke Skywalker found that the batteries for
Q108: Which one of the following is not
Q115: A NiMH battery uses _ as the
Q121: How long would it take to electroplate
Q127: How do fuel cells differ from traditional
Q129: Applying a current to a rechargeable battery
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents