The Following Reaction Occurs in Basic Solution Zn(OH)42-(aq) + NH3(aq)
A) Zn(s)
B) NO3-(aq)
C) OH-(aq)
D)
The following reaction occurs in basic solution. Identify the oxidizing agent. Note the reaction equation is not balanced. H2O( 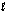 ) + Zn(s) + NO3-(aq) + OH-(aq) Zn(OH) 42-(aq) + NH3(aq)
) + Zn(s) + NO3-(aq) + OH-(aq) Zn(OH) 42-(aq) + NH3(aq)
A) Zn(s)
B) NO3-(aq)
C) OH-(aq)
D) H2O(l)
E) NH3(aq)
Correct Answer:
Verified
Q3: What is the oxidation number of chromium
Q15: When hydrogen reacts with a metal to
Q60: What is true when a battery (voltaic
Q63: How many kilograms of aluminum metal can
Q64: Neuron cells generate electrical signals by concentration
Q68: A pH meter uses an electrode arrangement
Q70: Bubbles will form on wires attached to
Q103: Methanol fuel cells depend on the
Q125: Chromium often is electroplated on other metals
Q128: If in using a lead-acid battery to
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents