What is the standard cell potential for this electrochemical cell? It consists of two compartments, labeled C1 and C2.
The temperature is 298 K.
C1 consists of a copper metal electrode immersed in a 0.30 M copper sulfate solution.
C2 consists of a copper metal electrode immersed in a 1.5 M copper sulfate solution.
Cu2+ + 2e- Cu, 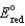 = +0.34 V
= +0.34 V
A) +0.34 V
B) -0.34 V
C) 0.0 V
D) +0.68 V
E) More information is needed to decide.
Correct Answer:
Verified
Q81: What is the cell potential for a
Q83: What kind of chemical reaction occurs at
Q84: The following reaction is called the
Q86: What is the cell potential for
Q87: An electrochemical cell at 298 K
Q102: Methanol fuel cells depend on the
Q133: The following reaction is called the
Q147: What is the change in free energy
Q148: What constitutes a standard hydrogen electrode?
Q159: How much work can be done using
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents