Ingestion of mercury, which is poisonous, often is treated using chelation therapy. One agent used in this therapy is dimercaprol, which is shown below. Explain why this compound has a high affinity for mercury atoms or ions and is able to tie these up in a complex so there is little free mercury to react with and disrupt the function of biological molecules. 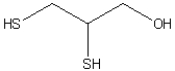
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q105: Unlike most transition metal compounds, those of
Q108: The pKa of hydrated iron(III) is 2.5,
Q109: Ingestion of mercury, which is poisonous, often
Q121: Write appropriate chemical reaction equations to show
Q122: Define the term polydentate ligand, and give
Q122: One of the following solutions is acidic.
Q124: What is the name of [Fe(NH3)5Br]2+?
Q126: Why are compounds of most of the
Q140: Draw and label the cis and trans
Q143: Explain why Ni(CN)42- is diamagnetic and NiCl42-
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents