In the following reaction in aqueous solution, the acid reactant is __________ and its conjugate base product is __________. CH3COOH + NH3 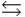 CH3COO- + NH4+
CH3COO- + NH4+
A) CH3COOH; CH3COO-
B) CH3COOH; NH4+
C) NH3; CH3COO-
D) NH3; NH4+
E) CH3COOH; H3O+
Correct Answer:
Verified
Q1: When [H+] = 1.0*10-7 M in water
Q2: Which of these is a strong acid
Q3: Ammonia (NH3) acts as a weak base
Q4: In the Brønsted-Lowry definition of acids and
Q4: In the following reaction in aqueous solution,
Q8: A solution with pH of 9.50 has
Q9: The base ionization constant Kb describes which
Q11: Which one, A-D, is not related to
Q62: The degree of ionization of a strong
Q70: The degree of ionization of a weak
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents