Phosphoric acid is a triprotic acid, ionizing in the following sequential steps: H3PO4 + H2O 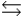 H2PO4- + H3O+ Ka
H2PO4- + H3O+ Ka
H2PO4- + H2O 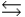 HPO42- + H3O+ Ka
HPO42- + H3O+ Ka
 HPO42- + H2O
HPO42- + H2O 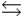 PO43- + H3O+ Ka
PO43- + H3O+ Ka
 What is the Kb expression for the base, sodium phosphate?
What is the Kb expression for the base, sodium phosphate?
A) 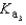
B) 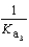
C) 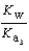
D) 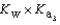
E) 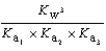
Correct Answer:
Verified
Q20: Carbon dioxide in the atmosphere dissolves in
Q21: A substance that can act as both
Q23: Bert and Ernie were determining the pH
Q26: Pure water at any temperature has _
A)
Q28: When values of Ka are small (e.g.,
Q43: In evaluating the pH of an aqueous
Q43: A solution with a pOH of 4.3
Q49: What is the pOH of a 0.20
Q60: What is the actual concentration of the
Q79: The degree of ionization _
A)increases with increasing
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents